Sen. Arthur Orr, R-Decatur, introduced a bill in the Senate Healthcare Committee last week that prevents occupational licensing boards from taking punitive actions against doctors who prescribe “off-label” drugs for the treatment of COVID-19.
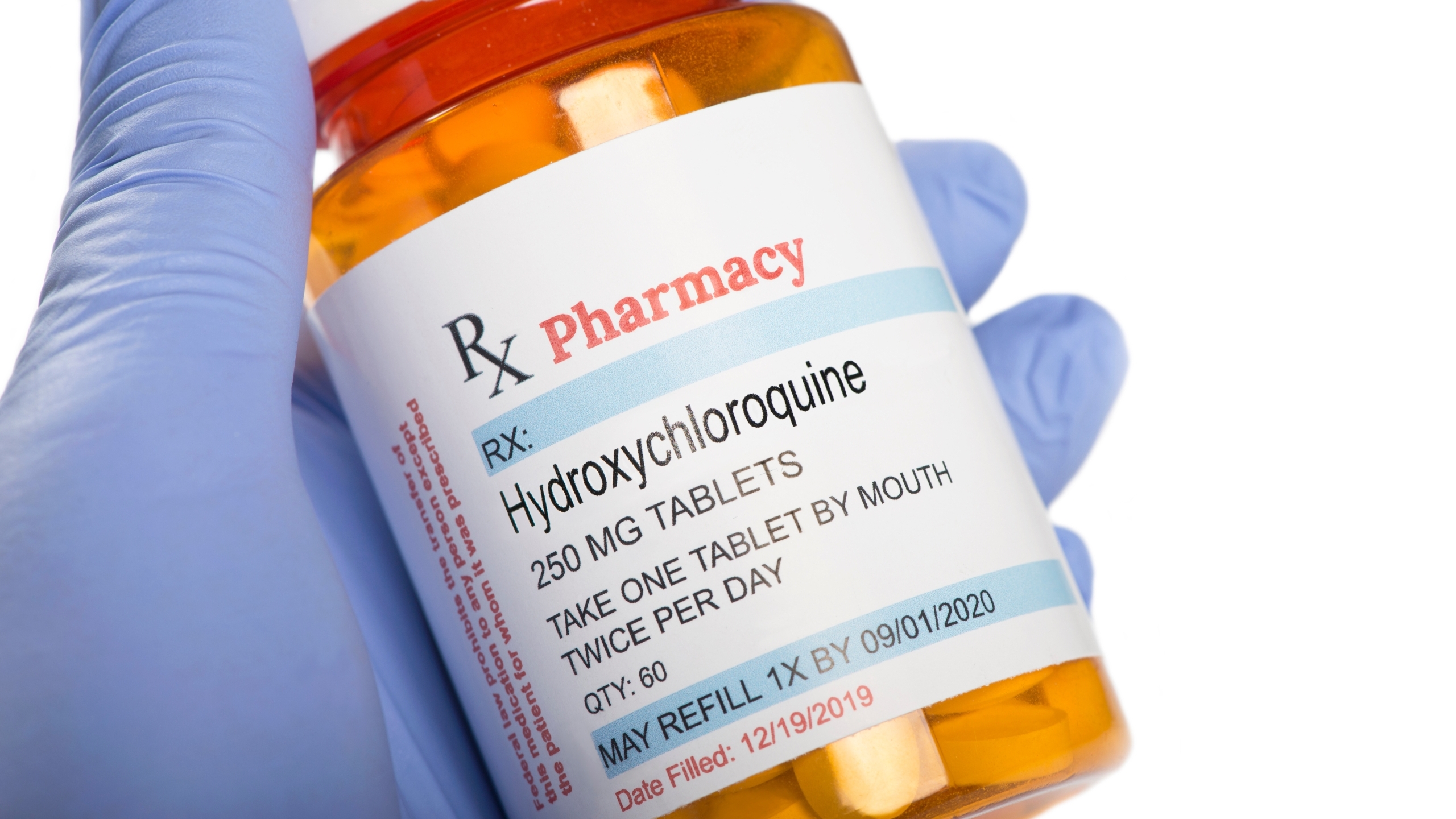
At a glance, this appears to be in response to the prescription of drugs such as ivermectin and hydrochloriquine, which have become a popular alternative despite major health organizations so far not finding enough evidence to show they are beneficial.
But the bill goes much further than just protecting the licenses of the few Alabama doctors who are prescribing the alternative treatments, it would also force healthcare facilities to provide these drugs, procedures or devices to any patient who requested them for the treatment of COVID-19.
The bill simply defines treatment for COVID-19 as “a procedure, protocol, drug, or remedy intended to prevent, mitigate, or treat COVID-19. The term includes the use of a drug, biological product, or device that has not been approved by the United States Food and Drug Administration (FDA) to treat COVID-19.”
The bill in its current form does not state who would determine that the “procedure, protocol, drug or remedy” is “intended to prevent, mitigate, or treat COVID-19” and upon what basis they would need to found the claim.
Orr laughed off a comment he relayed that doctors would be able to “prescribe Drano” for treatment. “We’re only talking about FDA-approved medications, just using them off-label” Orr said.
Although ivermectin and hydrochloriquine are perhaps the most prevalent medications being prescribed as “off-label” COVID-19 treatments, there appears to be no language in the bill that would prevent any other FDA-approved drug from being twisted into use as a COVID-19 treatment.
It’s unclear what, if anything, would prevent a patient from requesting opioids such as oxycodone or even fentanyl under the guise of COVID-19 treatment.
A few doctors said at a public hearing on the bill Wednesday that they need to maintain the freedom to prescribe “off-label” drugs to patients.
“So I feel like if you take a doctor’s autonomy to really practice, why do I study,” said Ryan McWhorter, a family practice doctor in Montgomery. “Why do I pour through research at night? I was up for hours into the wee hours trying to figure out what’s the best thing.”
However, while this bill would give doctors the autonomy to prescribe all kinds of alternative treatments for COVID-19, it would remove their autonomy to deny those alternative treatments.
It would also remove the autonomy of pharmacists to deny filling those prescriptions, something Sen. Tom Butler, R-Madison, took issue with in committee.
“While I think the physician ought to be able to do what he does, same time I do not think anybody should dictate in this bill what I can or cannot do as far as filling a prescription,” said Butler, who is a pharmacist. “I currently have the right to refuse to fill a prescription for whatever reason I may deem necessary.”
Butler indicated he would bring an amendment to that effect on the floor. However, he did not bring any issue with the bill limiting the autonomy of healthcare facilities.
The bill would require the patient to sign an informed consent form and release everybody involved from any liability, including the physician, the facility and the licensing board. A patient could not bring a cause of action against any entity for allowing the use of the off-label drug, but the bill creates a cause of action against healthcare facilities and pharmacies who refuse to administer an off-label COVID-treatment, and a cause of action against licensing boards for doctors.
The bill unanimously passed the Senate committee and could come before the full Senate with only seven legislative days remaining in the session. It is not clear when the bill will be placed on the calendar or whether it will reach the floor in time to pass both chambers of the Legislature.