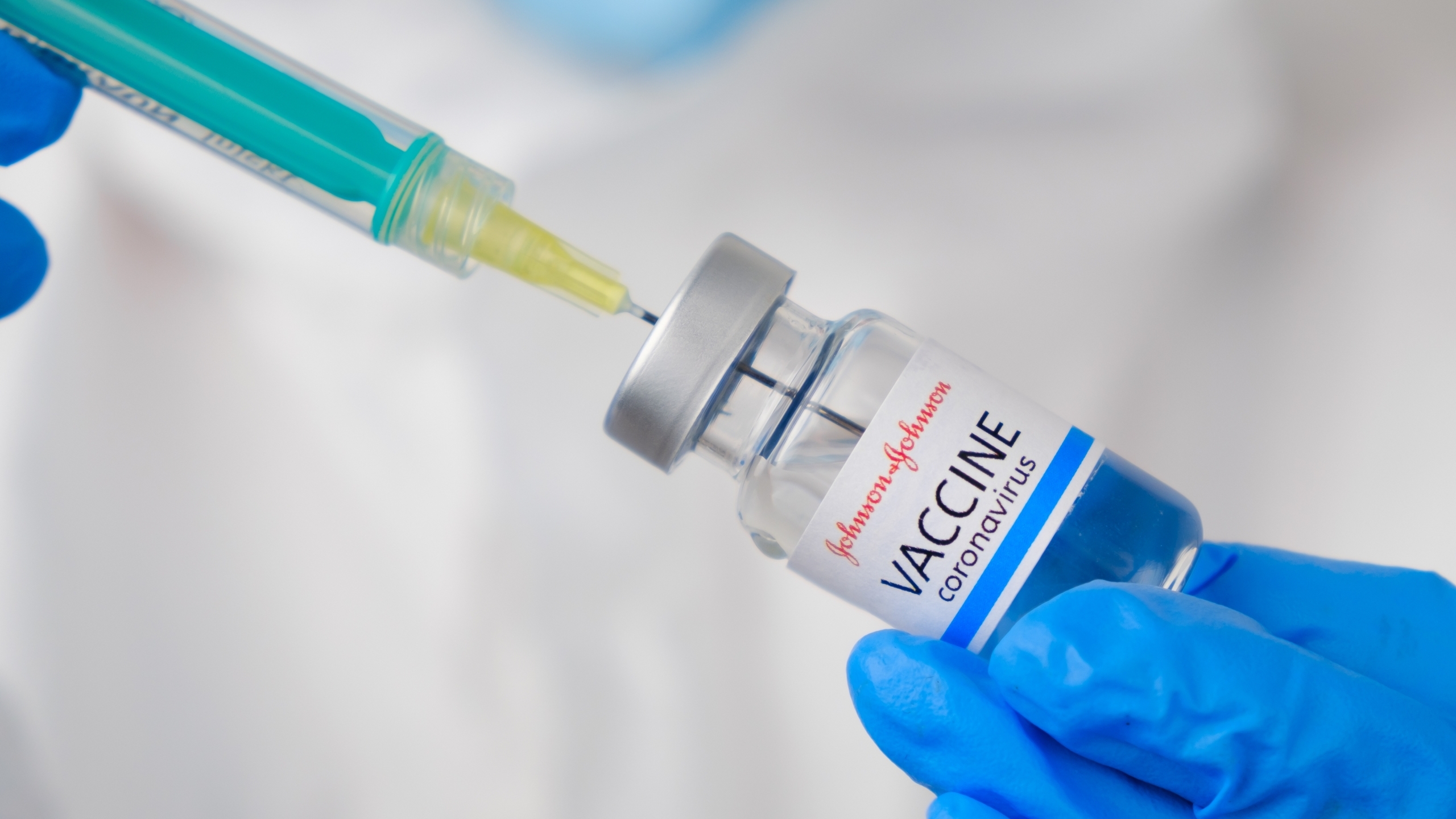
The Alabama Department of Public Health on Tuesday recommended that state providers return to using the Johnson & Johnson COVID-19 vaccine after receiving guidance from the federal government.
The CDC is investigating two new cases of extremely rare blood clots after the administration of the Johnson & Johnson vaccine, bringing the total number of such rare instances to 17 out of 8 million shots administered in the U.S., according to Reuters.
All but one of those persons with the rare blood clots were female and were primarily among women aged 18 to 49 years. No such rare blood clots have been identified after the use of the other two approved vaccines produced by Pfizer and Moderna.
The CDC and FDA on Friday recommended returning to the use of the Johnson & Johnson vaccine after a 10-day pause of its use.
“A review of all available data at this time shows that the J&J/Janssen COVID-19 Vaccine’s known and potential benefits outweigh its known and potential risks,” the CDC said Friday. “However, women younger than 50 years old should be aware of the rare but increased risk of this adverse event and that there are other COVID-19 vaccine options available for which this risk has not been seen.”
According to a CDC report released Tuesday, administering the Johnson & Johnson vaccine over the next six months at half the rate it was being used in April could save between 580 and 1,400 lives.
“The surveillance systems that are in place to monitor the safety of COVID-19 vaccines authorized for emergency use are effective, as demonstrated by both agencies’ quick work to identify and investigate these rare, but serious adverse events,” ADPH said in a press release Tuesday. “The FDA and CDC will continue with these efforts to closely monitor the safety of these vaccines.”
The ADPH notes that although the side effects are extremely rare, and no such side effects have been reported among Alabamians, anyone who develops them after receiving the Johnson & Johnson vaccine should immediately contact their health care provider and seek medical care.
Those side effects are a severe headache or blurred vision, shortness of breath, chest pain, leg swelling, persistent abdominal pain, or easy bruising or tiny blood spots under the skin beyond the injection site within three weeks of receiving the Johnson & Johnson vaccine.