The U.S. Department of Health and Human Services announced large-scale procurement of FDA-authorized rapid, point-of-care diagnostic test instruments and tests to be distributed to nursing homes in COVID-19 hotspot geographic areas in the United States.
This initiative is a one-time procurement of devices and tests targeted to facilitate on-site testing among nursing home residents and staff.
Through this crucial action, nursing homes will be able to augment their current capacity for coronavirus testing, bolstering their response and helping to prevent the spread of the novel coronavirus that causes COVID-19.
“Access to rapid point-of-care testing in nursing homes will further protect our Nation’s most vulnerable patients,” said Assistant Secretary for Health ADM Brett P. Giroir, M.D. “With the recent FDA Emergency Use Authorization of the BD Veritor system, combined with the earlier authorization of the Quidel Sofia and Sofia 2 systems, we now have the ability to provide more testing faster. This could not have been possible without the scientific investments made by these companies, advanced regulatory science from the FDA and other investments by the Federal government.”
Distribution will begin this week with nursing homes prioritized by the federal Centers for Medicare and Medicaid Services in their continuing effort to protect older adults.
Each nursing home will receive one diagnostic test instrument and associated tests. Following initial distribution, nursing homes can procure additional tests directly from the respective manufacturers.
Nursing homes must have the capability to screen and test residents, and test staff on a weekly basis or according to specific guidance by the state and local health departments. This procurement will also enable testing of visitors if appropriate for that facility.
“This new testing initiative is critical for keeping vulnerable older adults safe while delivering the quality of life they deserve,” said Seema Verma, CMS Administrator. “It gives nursing homes the ability to swiftly identify residents that need to be isolated and mitigate the spread of the virus. As one more tool in the toolbox, it represents an important step toward the long-awaited reunion of residents with their loves ones.”
Economic developer Nicole Jones said, “The Trump Administration has taken swift measures to protect our nation’s most vulnerable population. It is important that professionals across disciplines continue to collaborate to devise measures that will assist fellow Americans during this unprecedented time in human history.”
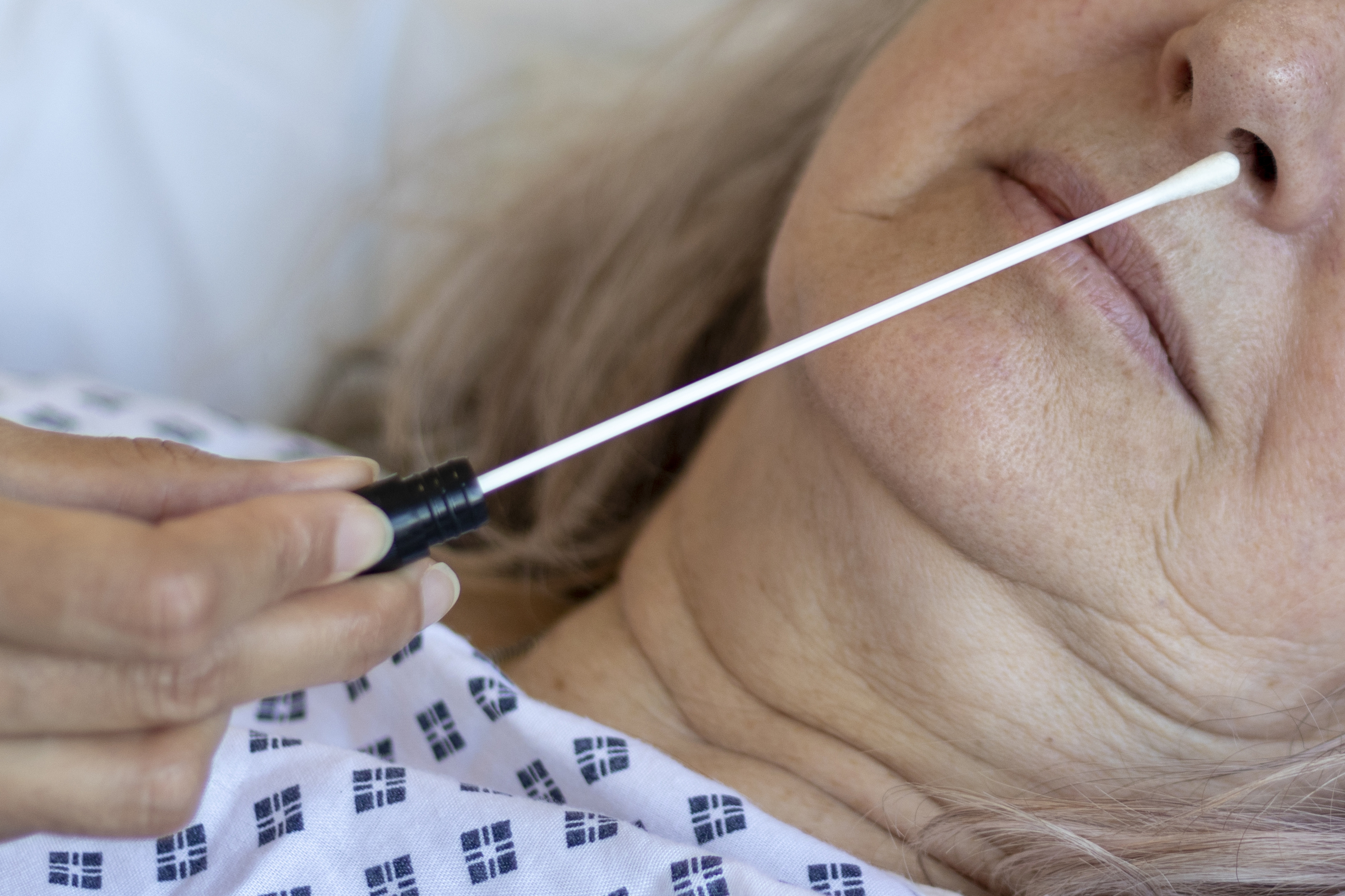
Both the Quidel Sofia and Sofia 2 Instruments and BD Veritor Plus Systems use antigen tests that can quickly detect fragments of proteins found on or within the virus by testing samples collected from the nasal cavity using swabs, providing results in minutes.
CMS says that antigen tests will play a critical role in the fight against COVID-19 and will be essential to reach the nation’s goal of testing millions of Americans each day.
The FDA has granted Emergency Use Authorization to two antigen detection tests: the Sofia SARS Antigen FIA manufactured by Quidel Corporation and the Veritor System for Rapid Detection of SARS-CoV-2 manufactured by Becton, Dickenson and Company. These tests may be slightly more likely to have a false negative result than molecular PCR COVID tests.
According to CMS, there are more than 200,000 confirmed or suspected cases of COVID-19 among nursing home residents.
Over 35,000 nursing home residents have died across the country from COVID-19 as of July 9, 2020. The CDC recommends that nursing homes perform baseline testing of all residents and staff, followed by regular screening and surveillance through routine testing to detect potential outbreak situations early and reduce morbidity and mortality.
At least 3,083 Alabama nursing home residents have already tested positive for the coronavirus as well as 1,953 employees of Alabama long term care facilities. At least 1,254 Alabamians have died from the COVID-19 global pandemic — many of them long-term care residents.
To date, 65,865 Alabamians have tested positive for the novel strain of the coronavirus, SARS-CoV-2.